Pharvaris Announces Positive Topline Data from RAPIDe-3 Pivotal Study Confirming Potential of Deucrictibant for On-Demand Treatment of HAE Attacks
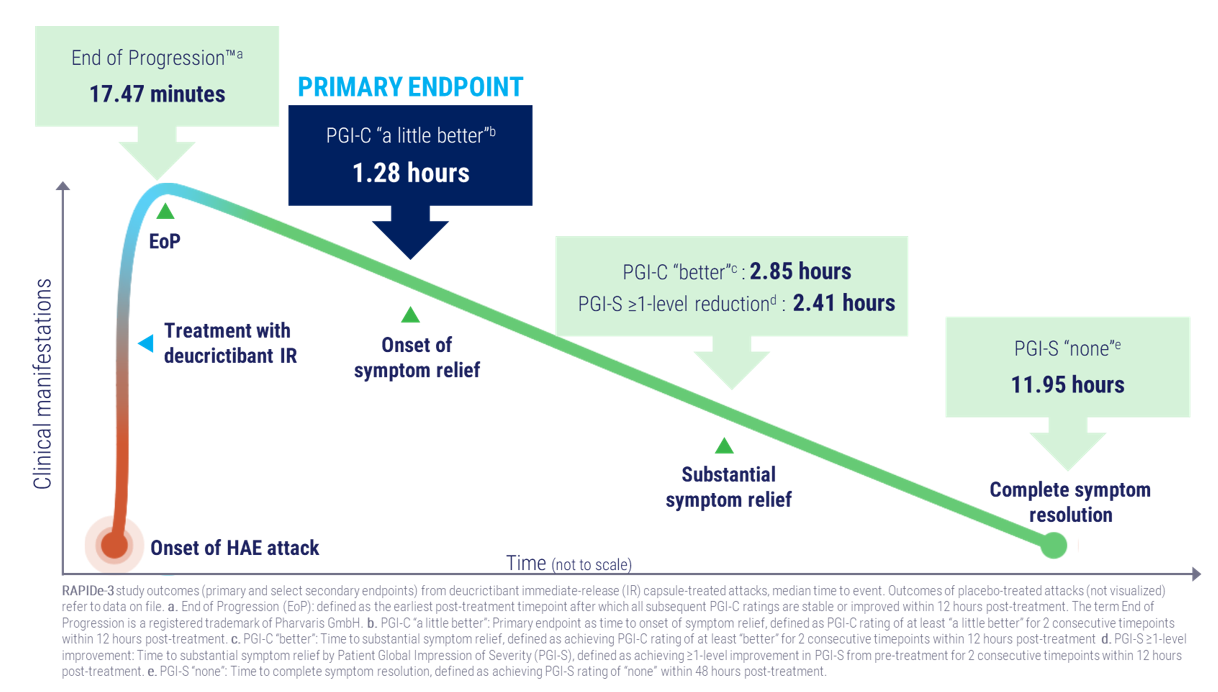
- Primary endpoint met; median time to onset of symptom relief achieved in 1.28 hours, significantly faster versus placebo (p<0.0001)
- All secondary efficacy endpoints met (p<0.0001), including End of Progression™1 (median 17.47 minutes) and complete symptom resolution (median 11.95 hours)
- Well-tolerated safety profile of deucrictibant confirmed
- Efficacy and safety outcomes consistent across all HAE subtypes represented (HAE type 1, HAE type 2, and HAE with normal C1 inhibitor) and varying attack severities and locations
- Pharvaris to host a conference call and webcast today at 8:00 a.m. EST
ZUG, Switzerland, Dec. 03, 2025 (GLOBE NEWSWIRE) -- Pharvaris (NASDAQ:PHVS), a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to help address unmet needs of those living with bradykinin-mediated diseases such as hereditary angioedema (HAE) and acquired angioedema due to C1 inhibitor deficiency (AAE-C1INH), today announced RAPIDe-3 pivotal data confirming the potential of deucrictibant's differentiated profile for the on-demand treatment of HAE attacks. The data from Pharvaris' first pivotal Phase 3 study will serve as the basis for marketing authorization applications, which are planned to be filed starting in the first half of 2026.
RAPIDe-3 Study Design and Results
The RAPIDe-3 (NCT06343779) global Phase 3, placebo-controlled study evaluated orally administered deucrictibant immediate-release (IR) capsule (20 mg) for the on-demand treatment of attacks in people 12 years and older with HAE. The study enrolled a total of 134 participants from 24 countries on six continents. The patient population studied included adolescents and adults; participants on and not on long-term prophylaxis (LTP); people with HAE type 1, HAE type 2, and HAE with normal C1 inhibitor; and attacks studied included those of varying severities and across all body locations. This makes this participant population the most representative and, based on prespecified endpoints, the RAPIDe-3 study the first-ever on-demand HAE study to be fully compliant with the Core Outcome Set recommended in the AURORA consensus.2 The primary endpoint and all 11 secondary efficacy endpoints, assessed sequentially under a multiplicity-control procedure, achieved statistical significance.
Compared to placebo, deucrictibant demonstrated compelling and differentiating efficacy:
- Faster median time to onset of treatment response:
- Time to onset of symptom relief by PGI-C3 at least "a little better": 1.28 hours versus >12 hours (p<0.0001)
- Time to End of Progression™: 17.47 minutes versus 228.67 minutes (p<0.0001)
- Shorter median time to substantial symptom relief:
- Time to substantial symptom relief by PGI-C at least "better": 2.85 hours versus >12 hours (p<0.0001)
- Time to substantial symptom relief by PGI-S4 ≥1-level improvement: 2.41 hours versus >12 hours (p<0.0001)
- Earlier complete symptom resolution:
- Median time to complete symptom resolution by PGI-S "none": 11.95 hours versus >24 hours (p<0.0001)
- Fewer attacks treated with second dose or rescue medication within 12 hours:
- 83.0% of attacks were treated with a single capsule of deucrictibant IR
- 93.2% of deucrictibant-treated attacks were treated without use of rescue medication
Marc A. Riedl, M.D., M.S., Professor of Medicine, Clinical Director of the U.S. Hereditary Angioedema Association (HAEA) Angioedema Center at the University of California San Diego (UCSD), and principal investigator in the RAPIDe-3 study, commented, "Bradykinin B2 receptor antagonism is a proven and effective mechanism for treatment of bradykinin-mediated angioedema. Injectable and oral on-demand therapies for HAE are available, however unmet medical needs remain. Effective, well-tolerated, and convenient acute treatment is an essential part of all HAE management plans due to unpredictable angioedema symptoms. The comprehensive and compelling outcomes of RAPIDe-3, specifically the fast treatment response and early complete symptom resolution, demonstrate the potential benefits of deucrictibant as an important on-demand treatment for people living with HAE."
Peng Lu, M.D., Ph.D., Chief Medical Officer of Pharvaris, stated, "I would like to sincerely thank the clinical study participants and their caregivers, the site investigators and staff, our study partners, the HAE community, and the Pharvaris team for their contributions to the RAPIDe-3 study. Together, we carefully designed and rigorously executed a study evaluating deucrictibant's ability to effectively address the unmet needs and high expectations of physicians, regulators, payers, and, most importantly, people living with HAE."
Dr. Lu continued, "These clinically meaningful and statistically significant results demonstrate deucrictibant's early-onset treatment response, fast symptom relief and resolution, and well-tolerated safety profile. This is an important step toward realizing deucrictibant's potential to offer control of bradykinin-mediated angioedema attacks. With these data in hand, our team is working diligently to prepare for regulatory filings to enable access to this promising therapy."
Berndt Modig, Chief Executive Officer of Pharvaris, added, "Since its founding, Pharvaris has spent the last decade pioneering science for patient choice. Deucrictibant combines the proven and effective mechanism of bradykinin B2 receptor antagonism in HAE with the convenience of oral administration. We are thrilled that RAPIDe-3 confirmed the profile of deucrictibant IR capsule established in Phase 2. If the CHAPTER-3 pivotal Phase 3 study confirms deucrictibant extended-release tablet as a long-term prophylactic of HAE attacks, deucrictibant could be the first and only oral therapy to offer control in both the on-demand and prophylactic treatment of bradykinin-mediated angioedema attacks."
In RAPIDe-3, deucrictibant was well tolerated with no treatment-related serious adverse events and no participants discontinuing treatment due to treatment-emergent adverse events. To date, the safety and tolerability profile was consistent with completed and ongoing studies of deucrictibant for the treatment of HAE attacks with no safety signals identified.
An open-label extension of deucrictibant for the on-demand treatment of HAE attacks, RAPIDe-2 Part B, is ongoing.
Pharvaris plans to present additional efficacy, safety, and patient experience data at upcoming medical congresses.
Pharvaris remains on track to submit a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) in the first half of 2026 for the on-demand treatment of acute attacks of HAE.
Conference Call
Pharvaris will host a live conference call and webcast to discuss the RAPIDe-3 study topline data in greater detail at 8:00 a.m. EST today via a live webcast; presentation slides may be accessed on the "Events and Presentations" page of the Pharvaris investor relations website. Participants interested in asking a verbal question during the Q&A may do so in the live conference call. An archived replay will also be available on the website for 90 days following the event.
About Deucrictibant
Deucrictibant is a novel, potent, orally bioavailable small-molecule bradykinin B2 receptor antagonist currently in clinical development. Deucrictibant is being investigated for its potential to prevent the occurrence of bradykinin-mediated angioedema attacks and to treat the manifestations of attacks if/when they occur by inhibiting bradykinin signaling through the bradykinin B2 receptor. Pharvaris is developing two formulations of deucrictibant for oral administration: an extended-release tablet to enable sustained absorption and efficacy as prophylactic treatment, and an immediate-release capsule to enable rapid onset of activity for on-demand treatment. Deucrictibant has been granted orphan drug designation for the treatment of bradykinin-mediated angioedema by the U.S. Food and Drug Administration, the European Commission, and Swissmedic.
About Pharvaris
Pharvaris is a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to help address unmet needs in bradykinin-mediated conditions, including all types of bradykinin-mediated angioedema. Pharvaris' aspiration is to offer therapies with injectable-like efficacy™, a well-tolerated profile, and the convenience of oral administration to prevent and treat bradykinin-mediated angioedema attacks. By delivering on this aspiration, Pharvaris aims to provide a new standard of care in bradykinin-mediated angioedema. Pharvaris is preparing global marketing authorization applications for deucrictibant immediate-release capsule as an on-demand treatment of HAE attacks, and a global pivotal Phase 3 study of deucrictibant extended-release tablet for the prevention of HAE attacks (CHAPTER-3) is ongoing with topline data anticipated in the second half of 2026. In addition, CREAATE is an ongoing Phase 3 study of deucrictibant for the prophylactic and on-demand treatment of AAE-C1INH. For more information, visit https://pharvaris.com/.
Forward Looking Statements
This press release contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and trials, and any statements containing the words "believe," "anticipate," "expect," "estimate," "may," "could," "should," "would," "will," "intend" and similar expressions. These forward-looking statements are based on management's current expectations, are neither promises nor guarantees, and involve known and unknown risks, uncertainties and other important factors that may cause Pharvaris' actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements. Such risks include but are not limited to the following: uncertainty in the outcome of our interactions with regulatory authorities, including the FDA; the expected timing, progress, or success of our clinical development programs, especially for deucrictibant immediate-release capsules and deucrictibant extended-release tablets, which are in late-stage global clinical trials; our ability to replicate the efficacy and safety demonstrated in the RAPIDe-1, RAPIDe-2, RAPIDe-3, and CHAPTER-1 Phase 2 and Phase 3 studies in ongoing and future nonclinical studies and clinical trials, such as CHAPTER-3, and CREAATE; the timing and outcome of regulatory approvals, including the timing and outcome of our planned submission of an NDA with the FDA in the first half of 2026 for the on-demand treatment of acute attacks of HAE; risks arising from epidemic diseases, which may adversely impact our business, nonclinical studies, and clinical trials; our ability to potentially use deucrictibant for alternative purposes, for example to treat C1-INH deficiency (AAE-C1INH); the value of our ordinary shares; the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, or any other product candidate that we may develop in the future; our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates; our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products; our ability to market, commercialize and achieve market acceptance for our product candidates; our ability to produce sufficient amounts of drug product candidates for commercialization; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others; our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws (including the Biosecure Act), our ability to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market conditions; disruptions at the FDA and other agencies; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and geopolitical conflicts; changes in regulations and customs, tariffs and trade barriers; and the other factors described under the headings "Cautionary Statement Regarding Forward-Looking Statements" and "Item 3. Key Information—D. Risk Factors" in our Annual Report on Form 20-F and other periodic filings with the U.S. Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While Pharvaris may elect to update such forward-looking statements at some point in the future, Pharvaris disclaims any obligation to do so, even if subsequent events cause its views to change. These forward-looking statements should not be relied upon as representing Pharvaris' views as of any date subsequent to the date of this press release.
1 The term End of Progression is a registered trademark of Pharvaris GmbH.
2 Petersen RS, et al. A Core Outcome Set for Efficacy of Acute Treatment of Hereditary Angioedema. J Allergy Clin Immunol Pract. 2024;12(6):1614-1621. doi:10.1016/j.jaip.2024.04.007.
3 PGI-C: Patient Global Impression of Change
4 PGI-S: Patient Global Impression of Severity
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ae4e7b97-9eee-4c93-b96a-9c39e55fd6ee

Contact Maggie Beller Executive Director, Head of Corporate and Investor Communications [email protected]
