Incannex Reports Positive Results from Phase 2 Clinical Trial of PSX-001 (Psi-GAD) for Generalised Anxiety Disorder
MELBOURNE, Australia and NEW YORK, Aug. 26, 2025 (GLOBE NEWSWIRE) -- Incannex Healthcare Inc. (NASDAQ:IXHL) ("Incannex" or the "Company") is pleased to report positive data from its Phase 2 clinical trial of PSX-001 (formerly Psi-GAD), a psilocybin-assisted psychotherapy treatment for Generalised Anxiety Disorder (GAD). The results confirm statistically significant and clinically meaningful improvements across every key endpoint assessed in the study, reinforcing PSX-001's potential as a best-in-class therapy for patients with moderate to severe GAD.
Trial Design Overview
The randomized, double-blind, placebo-controlled Phase 2 study enrolled 73 adult participants diagnosed with moderate to severe Generalised Anxiety Disorder. Participants were randomly assigned to complete two dosing sessions with either a 25 mg dose of synthetic psilocybin or placebo, administered in a controlled clinical setting as part of a proprietary psychotherapeutic protocol developed. All participants received equal hours of psychological support and preparation, ensuring that any treatment effect could be attributed to psilocybin itself and not therapeutic bias. Efficacy was assessed using a suite of validated clinical measures at multiple timepoints post-treatment, with the primary endpoint focused on change in HAM-A (Hamilton Anxiety Rating Scale) scores.
Exceptional Efficacy Results: Statistically Significant and Clinically Meaningful Improvements Across All Key Measures
The Phase 2 trial of Psi-GAD demonstrated a robust and consistent pattern of efficacy, delivering statistically significant and clinically meaningful improvements across all primary and secondary endpoints.
• HAM-A (Hamilton Anxiety Rating Scale): Patients treated with Psi-GAD achieved an average 12.8-point reduction in HAM-A scores from baseline, compared to a 3.6-point reduction in the placebo group. This difference was statistically significant (p<0.0001) and sustained across the 11-week follow-up period, indicating a rapid onset and durable treatment effect. As the trial's primary endpoint, the HAM-A outcome establishes a strong efficacy signal for Psi-GAD.
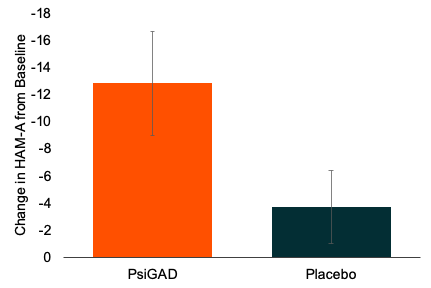
Figure 1. Change from baseline in HAM-A scores showing statistically significant and durable reduction in anxiety symptoms with PsiGAD compared to placebo.
• Clinical Response and Remission: Psi-GAD treatment resulted in a clinically meaningful response, corresponding to a reduction in HAM-A score from baseline of ≥50% in 44.1% of patients. A response rate more than four times higher than that of the placebo group. Of patients receiving PsiGAD treatment, 27% achieved full disease remission (HAM-A ≤7), five times higher than placebo.
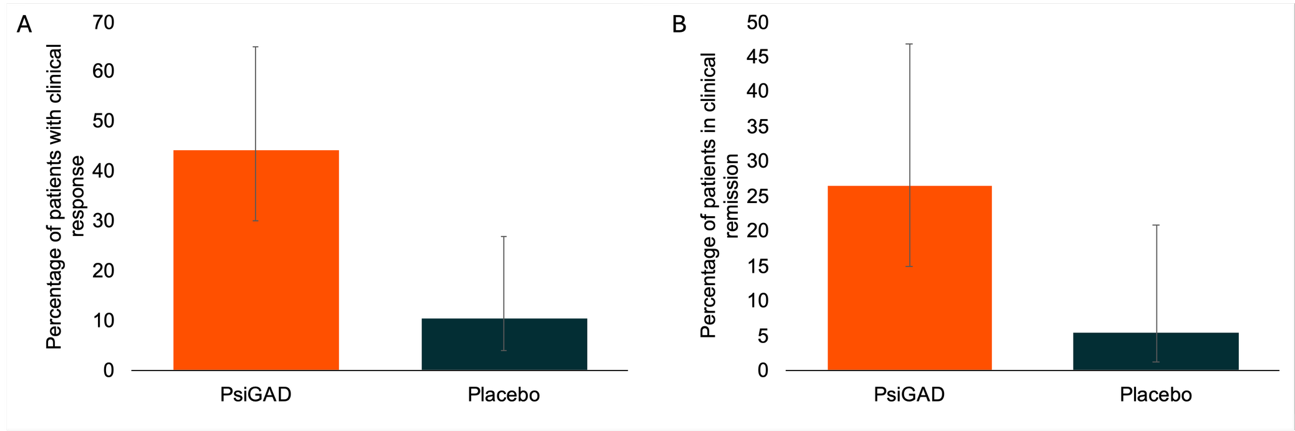
Figure 2. Rates of clinical response (≥50% reduction in HAM-A) and full remission (HAM-A ≤7) demonstrating substantial efficacy of PsiGAD vs. placebo.
• GAD-7 (Generalised Anxiety Disorder 7-item scale): The Psi-GAD group recorded an average 7.4-point reduction in GAD-7 scores, compared to a 3.5-point reduction for placebo. This difference was statistically significant (p<0.0004), confirming Psi-GAD's effect across multiple validated measures of anxiety severity.
• SDS (Sheehan Disability Scale): Patients in the Psi-GAD group experienced a 6.0-point reduction in SDS scores, versus 1.3 points in the placebo group—a statistically significant improvement (p<0.007). These results demonstrate a marked improvement in functional impairment across work, social, and family domains, which are often severely impacted in GAD.
• PHQ-9 (Patient Health Questionnaire-9): Psi-GAD also showed statistically significant antidepressant effects, with a 3.9-point reduction in PHQ-9 scores compared to just 0.3 points in the placebo group (p<0.005). This supports PSX-001's broad therapeutic utility in mood-related comorbidities.
• PWI (Personal Wellbeing Index): Quality of life, as measured by the PWI, improved by an average of 10.6 points in the Psi-GAD group versus 2.7 points for placebo—a statistically significant difference (p<0.002). These results reflect substantial and sustained enhancements in overall psychological wellbeing.
Safety and Tolerability
Treatment with Psi-GAD was well tolerated across the study population. No serious adverse events (SAEs) were reported, and only one of the 73 subjects withdrew from the trial during the 7 week treatment program. The majority of treatment-emergent adverse events (TEAEs) were transient, mild to moderate in nature, and consistent with the expected pharmacological effects of psilocybin. Importantly, there were no signs of increased suicidality, psychosis, or prolonged psychological distress—concerns often cited with psychedelic treatments. The controlled setting, comprehensive preparation, and therapist-supported integration sessions contributed to the overall safety of the intervention. These findings reinforce the strong risk-benefit profile of Psi-GAD and support its continued advancement into late-stage development.
Dr. Lou Barbato, Chief Medical Officer of Incannex, commented:
"These results speak for themselves—statistically significant, clinically meaningful, and consistent across every validated measure. Psi-GAD demonstrated a reduction in anxiety, improved mood, enhanced quality of life, and better day-to-day functioning. Importantly, the treatment effect was durable and observed across 11 weeks. These outcomes reinforce the power of our proprietary psychotherapeutic model and position Psi-GAD as a global leader in anxiety treatment innovation. We are also very pleased with the safety profile of Psi-GAD, particularly when considered alongside comparator programs in the market, which strengthens our confidence in both the clinical utility and scalability of this treatment."
Joel Latham, President and Chief Executive Officer of Incannex, commented:
"These are outstanding results for Incannex and a major milestone for our clinical pipeline. Alongside our strong cash position, the success of PSX-001 significantly de-risks another of our lead assets and further validates our strategy of developing innovative combination and psychedelic-based therapies. To deliver back-to-back positive Phase 2 results for both PSX-001 and IHL-42X is an exceptional achievement, and one that gives us tremendous confidence as we progress towards late-stage development and continue to build value for patients and shareholders alike."
Next Steps
The Company has an open Investigational New Drug (IND) application with the U.S. FDA and is preparing to initiate a multi-jurisdiction Phase 2 clinical trial. In parallel, Incannex continues to refine formulation strategies to ensure a robust and defensible intellectual property (IP) position. The Company is also exploring strategic partnerships to accelerate development and broaden global access to PSX-001 for patients with unmet needs.
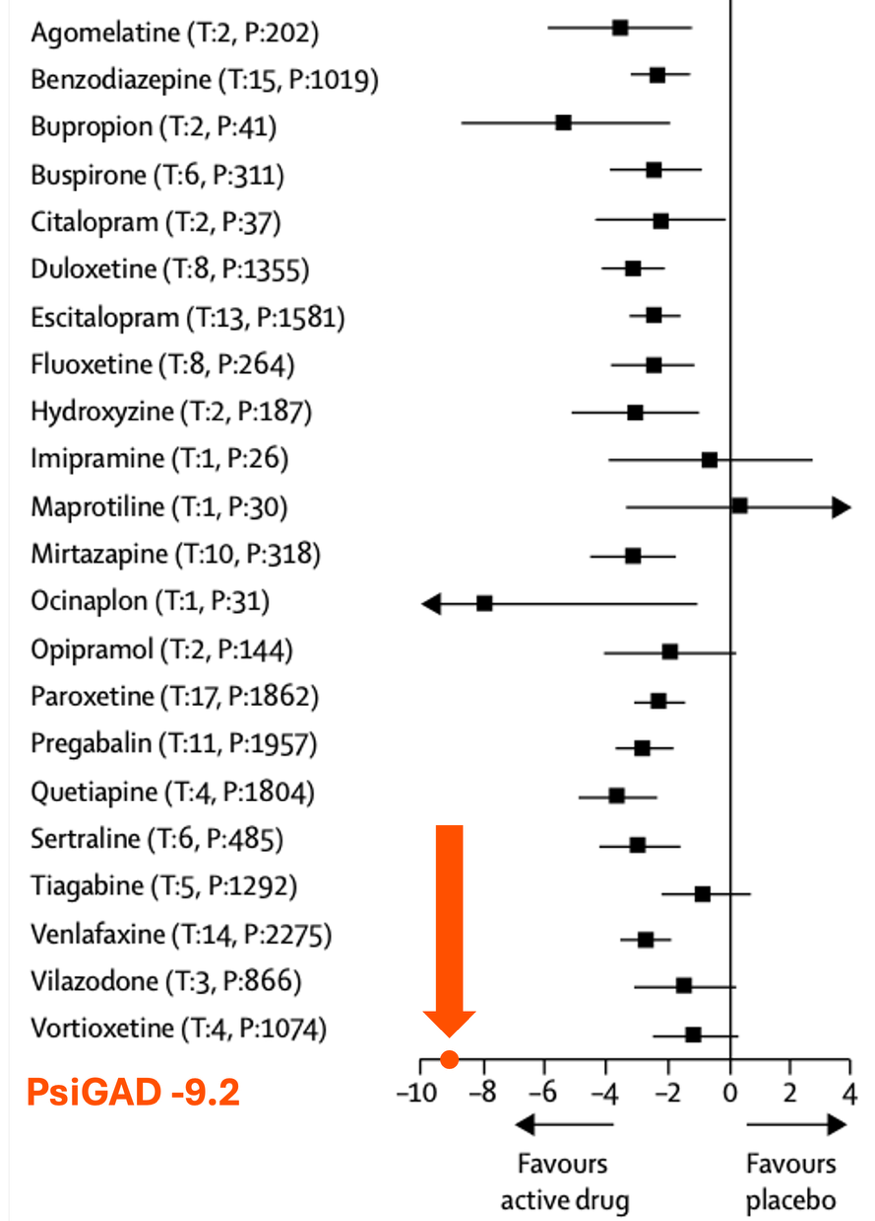
Figure 3. PsiGAD reduced anxiety scores to a greater extent than that reported for approved anxiety medications.
About Incannex Healthcare Inc.
Incannex is leading the way in developing combination medicines that target the underlying biological pathways associated with chronic conditions, including obstructive sleep apnoea, rheumatoid arthritis, and generalised anxiety disorder. The company is advancing three clinical-stage product candidates based on evidence-based innovation, and supported by streamlined operations. Incannex's lead clinical program, IHL-42X, is an oral fixed-dose combination of dronabinol and acetazolamide designed to target underlying mechanisms and act synergistically in the treatment of obstructive sleep apnoea. IHL-42X has successfully completed a Phase 2 clinical trial. PSX-001 is a synthetic psilocybin treatment for the treatment of generalised anxiety disorder. PSX-001 has recently completed a Phase 2 clinical trial with positive results, establishing it as a leading psilocybin-assisted therapy in development globally. IHL-675A is an oral fixed-dose combination of cannabidiol and hydroxychloroquine sulfate designed to act synergistically to alleviate inflammatory conditions, such as rheumatoid arthritis. IHL-675A is currently in a Phase 2 clinical development program. Incannex's programs target disorders that have limited, inadequate, or no approved pharmaceutical treatment options. For additional information on Incannex, please visit our website at www.incannex.com.
Forward Looking Statements
This press release contains "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements other than historical facts and relate to future events, future circumstances and Incannex's future performance. These statements are based on management's current assumptions, expectations, and beliefs. Examples of forward-looking statements in this press release include statements about, among other things: statements regarding potential future dilution; Incannex's opinions and estimates about the fundamentals and underlying value of its business relative to the trading price of its common stock; business strategy, future operations; Incannex's ability to execute on its objectives, prospects, commercial discussions or plans; evaluations and judgments regarding Incannex's research and development efforts and potential future commercialization, including any implications that the results (including qualitative patient-reported outcomes) of earlier clinical trials or interim or topline results will be representative or consistent with later clinical trials or their respective interim or final results; the potential benefits (including qualitative patient-reported outcomes) and safety of Incannex's drug candidates and the market opportunity for these candidates; and potential shareholder value. These forward-looking statements are subject to a number of risks and uncertainties, which may cause the forward-looking events and circumstances described in this press release to not occur, and actual results to differ materially and adversely from those described in or implied by the forward-looking statements. These risks and uncertainties include, among others: that current expense and cash resource estimates which may be ultimately be inaccurate and the Company may need to raise capital, including through the ATM, sooner than it presently anticipates; the continued availability of financing; Incannex's ability to raise capital to fund continuing operations and to maintain or potentially further improve its capital structure; Incannex's ability to maintain the listing of its shares of common stock on the Nasdaq Stock Market; the impact of any infringement actions or other litigation brought against Incannex; the success of Incannex's development efforts, including Incannex's ability to progress its drug candidates through clinical trials on the timelines expected and to obtain necessary regulatory approvals for commercialization of its product candidates; the effects of competition from other providers and products as currently existing or that may be developed in the future; that the market for its drug candidates may not grow at the rates anticipated or at all or that estimates for these markets may ultimately be incorrect; that Incannex may be unable to successfully execute upon any commercial discussions; Incannex's ability to comply with the various evolving and complex laws and regulations applicable to its business and its industry; and Incannex's ability to protect its proprietary technology and intellectual property; and other factors relating to Incannex's industry, its operations and results of operations. The forward-looking statements made in this press release speak only as of the date of this press release, and Incannex assumes no obligation to update publicly any such forward-looking statements to reflect actual results or changes in expectations, except as otherwise required by law. Incannex's reports filed with the U.S. Securities and Exchange Commission (SEC) including its annual report on Form 10-K for the fiscal year ended June 30, 2024, filed with the SEC on September 30, 2024, and the other reports it files from time to time, including subsequently filed annual, quarterly and current reports, are made available on Incannex's website upon their filing with the SEC. These reports contain more information about Incannex, its business and the risks affecting its business, as well as its results of operations for the periods covered by the financial results included in this press release. For additional information on Incannex, please visit our website at www.incannex.com.
Investor & Media Contacts
CORE IR
(212) 655-0924
[email protected]
[email protected]
Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/84e5d4b1-226c-4436-ac47-6e860bd7bacf
https://www.globenewswire.com/NewsRoom/AttachmentNg/3e07b30a-ecdd-4aa4-a6a5-5e569f791016
https://www.globenewswire.com/NewsRoom/AttachmentNg/f7109e12-d50d-4407-a1d4-7587681f151f

